
David Fajgenbaum became stricken by a mystery illness when he was a third-year medical school student. He soon suffered multiple organ failure and was placed in the intensive care unit. His condition grew so dire that his parents were told to say their goodbyes and a priest read him his last rites.
By then, doctors had diagnosed him as having idiopathic Castleman disease, part of a group of rare and poorly understood hematologic disorders where the immune system turns on the body. Fajgenbaum’s subtype is the deadliest and characterized by episodes of intense inflammation and multiple organ system dysfunction.
In a last-ditch effort to save Fajgenbaum, doctors administered an off-label therapy that saved his life. When he left the hospital more than four-months after being admitted, he left the work of Castleman disease research in the hands of others and continued down the path to becoming an oncologist.
But when he relapsed 15 months later, he took a much deeper dive into the world of Castleman disease research. He discovered while researchers around the world were studying the disease, none of them were working together. They were each using different terminology and classification systems, and none of them knew how the work they were doing related to the research others were conducting.
In August 2012, Fajgenbaum launched the Castleman Disease Collaborative Network (CDCN) with the hope of improving collaboration, developing a global research strategy, and driving research forward in the most cost-effective and efficient manner.
Last month, in a review article in the journal Emerging Topics in Life Sciences, Fajgenbaum, along with his organization’s COO Mary Zuccato, and senior scientific advisor Dustin Shilling, laid out their organization’s approach to research. In so doing, they provide a roadmap for other rare disease organizations wanting to avoid the pitfalls traditional approaches to research pose for rare diseases.
The traditional research model involves groups raising money, inviting investigators to apply for funds to use how they see fit, and a group of advisors choosing who should get those funds. The authors say this approach works well in disease areas where there is a competitive landscape and research materials are abundant.
But in rare diseases where there are limited qualified researchers interested in working on a specific disease, the chances that a highly qualified researcher will pose a high-impact research project becomes much more unlikely.
Instead, the approach CDCN lays out involves identifying the stakeholder community, having the community prioritize a research agenda, and recruiting experts to conduct the studies.

Photo: CDCN’s David Fajgenbaum, Mary Zuccato, and Dustin Shilling
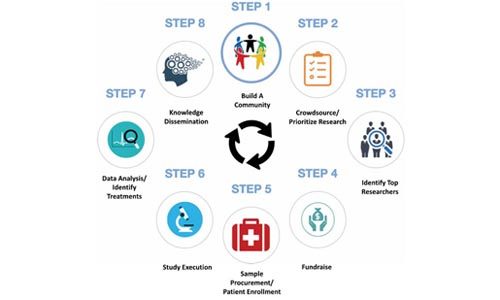
Building on the work of a number of rare disease organizations, the authors describe an eight-step approach. The process also involves raising money for the studies that need to be conducted, recruiting patients and patient samples, and assisting with the execution of studies by providing project management and scientific advice.
The two final steps involve data analysis with a focus on identifying potential treatment (particularly already approved therapies that could be repurposed) and disseminating the information by helping publish and distribute the findings.
“This final step ensures that the community, described in Step 1, is well informed about scientific progress and therefore better able to identify and prioritize the next round of high-impact research,” the authors write. “After disseminating findings throughout the community, the cycle continues: more individuals join, new research ideas are shared (inspired by the findings), and greater progress towards the CDCN’s mission is accomplished.”
Prior to the CDCN’s founding, the authors note that there were few advances in the understanding or treatment of Castleman disease. There was no foundation focused on advancing research, limited collaboration between researchers, no centralized registries or biobanks, and few published studies. What studies existed had limited sample sizes, inconsistent terminology for subtypes of the disease, and different approaches to stratification that made comparisons between studies difficult. The combined effect of this was to slow the understanding of the disease and the identification of potential targets and therapies.
The CDCN’s approach has changed the landscape for Castleman disease. It has made significant progress toward the goal of finding therapies for all of Castleman disease. Since its founding in 2012, the CDCN has connected and engaged more than 500 physicians and researchers and more than 10,000 patients and family members. With these communities it has developed and carried out an international research agenda that has supported 23 research projects with samples, study coordination, and/or data analysis. It has also funded 19 projects and facilitated the publication of more than 20 research papers, including a uniform Castleman disease classification system and multiple case series describing more than 400 patients.
Additionally, it has developed the first-ever diagnostic criteria for idiopathic multicentric Castleman disease and the first-ever treatment guidelines for this form of the disease. CDCN members also served as investigators on the clinical trials that led to the first-ever FDA-approved therapy for idiopathic multicentric Castleman disease.
To learn more about CDCN’s work, please read the full report, NEXT: Imagining the Future of Rare Disease.
Listen to our 2015 interview with CDCN’s David Fajgenbaum where he discusses the CDCN’s collaborative research model.

Stay Connected
Sign up for updates straight to your inbox.
