This Global Genes™ Toolkit will provide you with an overview of how new drugs are developed and approved in the U.S. and also what the differences are when developing new drugs for rare diseases. This Toolkit is one in a Global Genes series on drug development, From Molecules to Medicine, with others in the series providing more detail on each step of the process.
In this Toolkit, we will take a look at each of the steps in the drug development process, outline differences in drug development for rare diseases, and discuss why now, more than ever before, the rare disease community can be optimistic about the development of new treatment. Our goal is to provide you with the information you need to participate more effectively in the development of treatments for rare diseases.
The Drug Development Process
Drug development is a complicated process. The science behind new medical treatments is necessarily full of trial and error, as researchers must explore many possibilities to identify the most promising, safe and effective treatments. The process can be lengthy, requires significant funding and is strictly governed by regulatory agencies to ensure that new drugs will be safe and effective and have the utmost benefit for patients.
What is the basic process?
The drug development process begins with drug discovery—scientists explore many chemical and biological compounds to identify those with the potential to treat a particular disease or impact a particular biological function. Scientists then go on to study the compound as a potential treatment in the lab and in animals. If these studies show the drug may be effective and safe, an application if filed with the regulatory agency for approval to begin clinical trials. In the U.S., this application is an Investigational New Drug (IND) application. The drug is next studied in people who volunteer for clinical trials. Clinical trials are done in three phases, or steps, each of which answers a specific research question. Once all three clinical trial stages have been completed successfully, meeting strict standards for safety and effectiveness, a potential new treatment can be submitted to the regulatory agency for approval. In the U.S., this is done through a New Drug Application (NDA). Approval of an NDA means that doctors can now provide the new treatment to patients. After approval, the FDA continues to monitor new drugs to make sure there are no side effects or issues that were not found during clinical trials. In the U.S., the FDA may require that post-marketing surveillance studies, also known as Phase 4 studies, be done after a drug is approved for marketing to get additional information on the drug’s risks and benefits or best use.
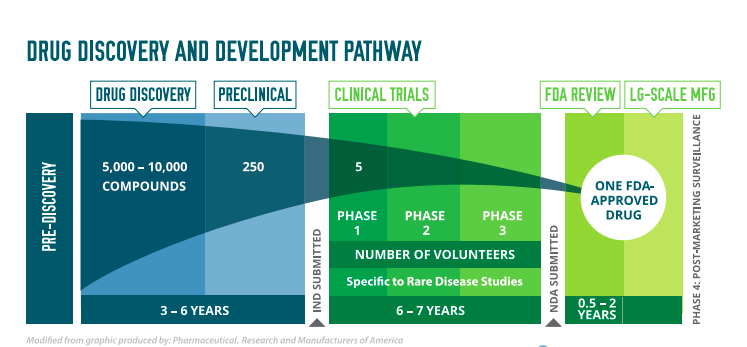
STEP 1 – DRUG DISCOVERY
In drug discovery, scientists are looking for chemical or biological compounds that may have an impact on a particular disease or mechanism. For example, if a disease is known to cause certain proteins to be over-produced, scientists may try to discover a new compound that prevents that from happening. Drug discovery happens in academic research labs and within biotech and pharma companies.
STEP 2 – PRECLINICAL DEVELOPMENT
Once a promising new compound is found, preclinical testing can begin. At this stage, many laboratory and animal tests are completed to see whether the compound is safe enough to be used in people. Before testing any possible new drug in people, researchers must find out whether it has the potential to cause serious harm, also called toxicity. Usually, preclinical studies are not very large. However, these studies must provide detailed information on dosing and toxicity levels. After preclinical testing, researchers review their findings and decide whether the drug should be tested in people. If researchers want to take the next step and begin testing in people, they must submit an Investigational New Drug Application (IND) to the FDA. The FDA reviews the IND to make sure that the people who volunteer for the clinical trials will not face unreasonable risks. The IND includes detailed information on the safety of the new drug, the plan for clinical trials in people, and details on how the drug will be manufactured. After the IND is approved, the drug can be studied in people in Clinical Trials.

Step 3 – CLINICAL RESEARCH
Clinical Trials or Studies are usually run in three phases before marketing approval. Sometimes a fourth phase is required by regulatory agencies to monitor a new drug once it has been approved. Trials require collaboration and coordination of many different teams including clinical investigators, the patients who volunteer to participate, and the statisticians, coordinators and managers who make sure that the trial is conducted according to regulations during the length of the study. The four phases of clinical trials are described briefly below. More detailed information is available in the From Molecules to Medicine: Clinical Research toolkit.
Phase 1 Trials
Researchers test an experimental drug or treatment in a small group of people for the first time. The researchers evaluate the treatment’s safety, determine a safe dosage range, and identify side effects. Phase 1 trials determine the highest dosage of the drug that can be administered without causing serious harmful effects. Healthy volunteers (people who do not have the disease that the drug will treat) usually participate in phase 1 studies. However, for certain diseases, volunteers who have the disease may be recruited instead.
Phase 2 Trials
The experimental drug or treatment is given to a larger group of people to see if it is effective and to further evaluate its safety. Phase 2 studies determine if the drug will help patients who have the disease. If the drug is found to be effective in this small number of patients, then much larger phase 3 studies may begin. Phase 2 trials are often “placebo-controlled, randomized and double-blinded” See below for what these terms mean:
Phase 2 Trials – Definitions
• Placebo-controlled – Some patients in the trial receive the drug; others receive a lookalike product that has no effect, called a placebo
• Randomized – Patients are picked at random to get one of the study treatments
• Double-blinded – Neither the patients nor researchers know who has received which treatment – drug or placebo, until the study is over
Phase 3 Trials
The experimental study drug or treatment is given to large groups of people. Researchers confirm its effectiveness, monitor side effects, compare it to commonly used treatments if they exist, and collect information that will allow the experimental drug or treatment to be used safely. Phase 3 studies usually take place at many study sites or clinics, take place over several years, and are usually randomized, double-blinded studies.
Patients can help move clinical trials for rare diseases forward by asking their doctors to help them find trials, sharing trial information with their communities and volunteering to take part. A Global Genes Toolkit is available on Clinical Research.
Phase 4 Trials
Post-marketing studies, which are conducted after a treatment is approved for use by the FDA, provide additional information including the treatment or drug’s risks, benefits, and best use.
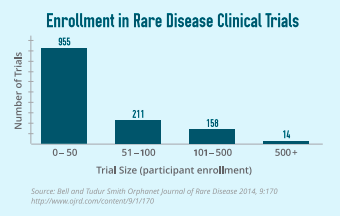
Rare Disease Clinical Trials
While drug development can be a lengthy endeavor for any disease and requires participation in each phase of clinical trials, for rare diseases, the number of participants in those trials in some circumstances can look very different from a trial for a common condition such as diabetes or heart disease.
It is essential to demonstrate safety and effectiveness throughout the clinical trial process. With rare diseases it can be possible to demonstrate safety and effectiveness using small enrollment clinical trials that has been accepted by FDA and has led to approvals. However, the need to work with regulators to accept smaller clinical trial enrollment for rare disease treatments continues to be an important issue for the rare community. Following are some examples of relatively small clinical trials for orphan therapies. Human Fibrinogen Concentrate (HFC) treats congenital fibrinogen deficiency, was approved in 2009 for this rare disease, which had no treatment. The prevalence of the disease is around 300 people in the U.S. The approval of HFC was a result of a pivotal study of 15, a retrospective clinical study of 100, and two additional studies of 12 and 6 patients.
Elosulfase is an enzyme replacement therapy, which treats MPS IVA, a rare lysosomal storage disorder with a prevalence of 500 – 800 patients in the U.S. The therapy was approved with one pivotal trial of 176 patients followed by open-label extension where all patients received elosulfase. The entire program comprised six clinical trials which also included a Phase 1/2 with 20 participants and two ancillary Phase 2 trials with 35 participants.
Sources: Human Fibrinogen Concentrate Briefing Document (BLA 1253 i 7/0), 2009; Rare Disease Clinical Trials, Anne Pariser, MD, Office of Translational Science, CDER, FDA, November 4, 2014.
STEP 4 – REGULATORY REVIEW & APPROVAL
After completion of the first 3 phases of a clinical trial, the drug company analyzes the data. If the data show that the drug is effective and safe, the company submits a New Drug Application, or NDA, to the FDA. An NDA may be more than 100,000 pages long and contains data gathered from all of the preclinical and phase 1-3 studies, plus analyses of that data. An NDA will include pharmacokinetics or what the body does to a drug—the movement of drug into, through, and out of the body, as well as information on how the drug is manufactured.
Review of an NDA by the FDA may take 10 to 12 months. During that time, scientists at the FDA go through all of the information in the application to see if there is solid evidence that the drug is safe and effective. The FDA compares the benefits of the drug to the risk for patients taking it. The FDA also decides what information should be included in the package insert to instruct doctors about how to use the drug and for which patients. Finally, the FDA ensures that the drug is being manufactured to a high quality. If the drug is approved, then manufacturing on a large scale begins and companies can market the drug to doctors and patients.
Phase 4: After Approval – Monitoring Safety
After approval of a drug, its long-term safety may be studied in a phase 4 post-marketing study. Phase 4 monitoring of a new drug allows the reporting of any new, potentially harmful effects not seen during the other phases of clinical studies. This includes information about effects of the drug that are rare or unexpected, that last a long time, or that arise after the drug is taken for a long time. Any harmful effects found after a drug has been approved can be reported by patients and doctors through the FDA’s MedWatch voluntary system. When important new risks are found, the public is informed through letters and public health advisories. Usually the risks are added to the drug’s package insert.

Differences In Orphan Drug Development And Approval
Rare disease patients and advocates need new treatments and drugs fast! There are 7,000 rare diseases and yet there are only around 400 drugs approved by the FDA to treat them.
To add to the urgency, the National Institutes of Health estimates that 50% of people affected by rare diseases are children, making rare diseases one of the most deadly and debilitating for children worldwide.
For many years, rare diseases were not attractive to biotech and pharmaceutical companies as the diseases were extremely difficult to treat and the numbers of patients affected were extremely small. Rare disease did not fit the blockbuster drug development model, as the numbers of patients affected with rare conditions were too small.
The Orphan Drug Act of 1983— Recognizing the scarcity of medicines to treat rare diseases and the unique challenges with developing drugs and therapies for very small patient populations, the Orphan Drug Act of 1983 provided incentives to companies developing rare disease treatments. Over the last 30 years, about 400 medicines representing 447 separate indications have been approved to treat rare diseases, compared to fewer than 10 in the 1970s.
Incentives provided by the government to rare disease drug companies today include:
• Seven years of exclusive marketing, which means that no other drug company can receive approval of the same drug for the same disease, unless the new drug can demonstrate greater safety, greater efficacy, or a major contribution to patient care.
• A 50 percent tax credit for qualifying research expenses
• Grants supporting the development of any drug used to treat a rare disease
• Companies developing new drugs for rare diseases do not have to pay the same fees as companies developing drugs for common diseases.
• Special assistance from the FDA regarding studies to support a rare disease. The FDA provides extensive and timely guidance to drug companies about the data that are needed for approval of a drug to treat a rare disease.
FDA has also put in place special processes that can speed up the approval of treatments for rare diseases:
Fast-track status . With fast-track status, the development and review processes move more quickly, if the drug is for a serious disease and “fulfills an unmet medical need” (that is, the disease does not already have an approved treatment or the drug may work better than existing drugs). When a drug is given fast-track status, the FDA and the drug company communicate with each other early and often during the development process to address any questions and problems that arise. Fast-track status also makes the drug eligible for accelerated approval and priority review

Accelerated Approval. With accelerated approval, development and approval are sped up, if the drug is for a serious condition and fulfills an unmet medical need. Ordinarily, approval is based on the benefits of the drug, but with accelerated approval, it can be based on a “surrogate endpoint.” A surrogate endpoint such as a certain laboratory test result that may predict that there will be a benefit, but that is not by itself a measure of a benefit.

Priority Review. With priority review, the FDA takes action on an application within six months, rather than the typical 10 months of a standard review. Priority review may be allowed for a drug that is an important advance in the treatment of a disease such as a drug that has increased effectiveness, has fewer harmful effects, or shows effectiveness and safety in a new group of patients. There is no difference in the standard for approval or in the quality of the evidence between standard review and priority review.

Breakthrough Therapy Designation. If a drug is designated by FDA as breakthrough therapy, the agency will expedite its development and review. A breakthrough therapy is a drug that is intended to be used by itself or in combination with one or more other drugs to treat a serious or life-threatening disease or condition, and where clinical evidence indicates that it may be a substantial improvement in at least one clinically significant endpoint over available therapies.
Rare Pediatric Disease Priority Review Voucher. The FDA will grant a priority review voucher (PRV) to sponsors upon approval of an eligible rare pediatric disease treatment (either a New Drug Application or Biologics License Application). The sponsor can choose to redeem the voucher toward the FDA review of a future drug in its pipeline, or sell the voucher to another company. The use of a priority review allows for a shortened FDA review timeline—six months for a priority review versus 10 months for a standard review. FDA has awarded six Rare Pediatric Disease Priority Review Vouchers to date and four have been sold, the latest for a record-setting $350 million in August 2015, providing a powerful incentive for rare disease drug developers and attracting investment into the orphan drug space. Use of a PRV does not guarantee approval, therefore, the unsuccessful use by a company of a voucher is a costly failure. One other challenge is that the program is set to end March 17, 2016, unless Congress takes action

How are Genetic & Genomic Technologies Changing Drug Development?
Of the 7,000 rare diseases identified worldwide, approximately 80 percent are caused by genetic changes. Until recently, drugs have been developed with the idea that each drug works pretty much the same in everybody. But advances in genetic and genomic research and technology has changed that “one size fits all” approach and opened the door to more personalized approaches to using and developing drugs.
For patients with rare diseases, the ability for researchers to study genetic data to identify the cause of a disease can provide a diagnosis were none was possible before. Genetic research can also help identify new approaches to treatment—understanding how changes or mutations in a gene impact the body can help researchers find new paths to treating the disease. Ultimately, gene therapy may be used to cure disease by replacing faulty genes with genes that function correctly.
While we are still at the very beginning of genetic medicine, this new understanding is already helping rare disease patients and families find a diagnosis, understand their risks for inherited disease, support geneticbased research by sharing their genetic data and family histories and continues to drive hope for treatments and ultimately cures for all rare disease patients.
How Can Rare Disease Patients Participate in Drug Development?
Patients with rare diseases and their families used to deal with their challenges alone. The rare disease community was built by parents and other family members with sick loved ones who have gone through a long and difficult process to get a diagnosis. Because of their dedication and strength, today the rare disease community is a force made up of not only patients and their families and caregivers, but also patient advocate groups, clinicians, and academic researchers. More recently, social media has helped connect people within the rare disease community with each other, as well as with others. This strong community is an asset to drug companies in many ways—for example, by increasing awareness of the need for new treatments for rare diseases, and by providing access and connections to patients with rare disease, which helps drug companies recruit patients for clinical trials.
While government plays a critical role in helping to offer incentives to industry, the fact is patient advocates play a far more important role, especially when treatment options are just starting to be identified and understood. By raising awareness of the disease, taking steps to capture real-world data as well as organizing first-ever clinical trials, many patients and family advocates are leading this new fight. This generates the formation of initial patient groups, which benefits both providers and pharmaceutical companies in understanding the needs of patients and caregivers. This also accelerates the commercialization of medications as well as treatment protocols based on input from the community.
The ability to target rare disease for small patient populations is further advanced with the help of wearable devices and mobile technologies. Many patients and families are helping to capture and report this data for wider research and usage.
Patients and family caregivers are finding the courage to look to the future, regardless of the many road-blocks and challenges ahead. By coming together and being proactive in organizing data collection standards, they are in a better position to identify opportunities to help reduce the time from diagnosis to treatment.
Conclusion
The rare disease community is growing, and more treatments are being developed and approved than ever before. This is an exciting time for the discovery of new therapies for rare diseases—a time that offers more hope than ever before.

Stay Connected
Sign up for updates straight to your inbox.