Innovative Ideas from Next Generation Change-Makers

The RARE Drug Development Symposium, hosted by Global Genes and the Orphan Disease Center of the University of Pennsylvania, equips advocates with the knowledge, skills and connections they need to advance therapy development for their communities. This year’s theme is Innovative Ideas from Next Generation Change-Makers.
Can’t make it to Philadelphia?
Have questions about this event? Email [email protected].
* Global Genes does not sell conference/participant information or user data.

What to expect from 2024 keynotes and sessions:
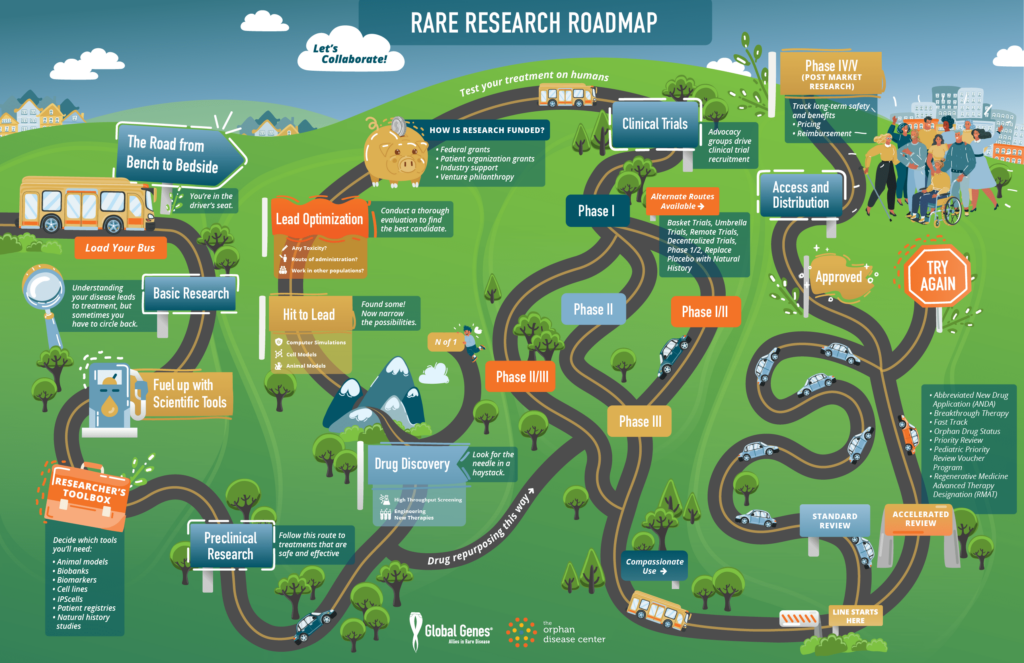
* Interactive Groups, Resource Fair & RARE Research Roadmap
* How Advocates are Shifting the Paradigm to Push Past Limits
* Rethinking Clinical Trials
* Patient-Led Data Initiatives
Rare Drug Development Happens Year-Round
Global Genes provides educational resources to help advocates and organizations become better equipped to work with researchers and industry partners throughout the drug development process.
Rare Research Roadmap: This toolkit was designed to help advocates, individuals, families, and organizations better understand some of the potential routes to treatment and some of the key concepts that are part of these processes to work with researchers and industry partners throughout the drug development process.
Event Speakers

Opening Keynote: Shifting the Paradigm to Push Past Limits
April 30, 9:00 – 9:45 am ET
Advocates are dramatically altering the landscape of rare research and reducing the timeline for rare disease therapy development. What is it that allows some orgs to move faster, be more nimble, use resources effectively and blaze new paths? Is there a matrix that can help you determine what will work for you?

Closing Keynote: Fireside Chat with Dominique Pichard
May 1, 2:45 – 3:45 ET
What new solutions are emerging to accelerate translational research in rare disease? Global Genes CEO chats with Dominique Pichard, Director of the Division of Rare Diseases Research & Innovation at NCATS, about how her unique experience as a physician, rare Mom and advocate will inform her approach to leading innovation in rare disease research at NCATS.
More speakers for the 2024 RARE Drug Development Symposium are being added — check back soon to see what sessions they will be presenting!
Betty Cabrera, MPH
Gene Therapy Initiative
Betty Cabrera, MPH
Gene Therapy Initiative
Director of Research Engagement and Operations
Gene Therapy Initiative
Session: What Will $100K Buy You? Emerging Commercial and Non-Profit Financing Models
In her current role with the Gene Therapy Initiative, Betty Cabrera is helping shape a prominent gene therapy research program. She has extensive experience advancing cell and gene therapy investigational product development, having contributed to establishing one of the first clinical research units in California dedicated to testing these products. Betty appreciates the needs of patients with rare genetic diseases and their families and is optimistic about future improvements in well being.
Jeff D’Angelo
CHAMP1 Research Foundation
Jeff D’Angelo
CHAMP1 Research Foundation
President and Founder
CHAMP1 Research Foundation
Session:
Partnerships in Action
RARE-X Exchange Forum*
Jeff D’Angelo is the Founder and President of the CHAMP1 Research Foundation. Since his son’s diagnosis his motivation has been to accelerate research in hopes for a treatment and a cure for kids whose lives have been affected by CHAMP1 disorders.
*Please note that RARE-X Exchange Forum sessions require an additional registration fee.
Scott Demarest, M.D.
Children’s Hospital Colorado
Scott Demarest, M.D.
Children’s Hospital Colorado
Associate Professor
Dept. of Pediatrics and Neurology CU School of Medicine & Children’s Hospital Colorado
Session:
Integrating Clinical and Patient Reported Data
RARE-X Exchange Forum*
Dr. Scott Demarest is an associate professor in the Department of Pediatrics, Division of Neurology. He is board certified in Child Neurology and Epilepsy. His clinical practice and research focus on the evaluation and treatment of neurogenetic conditions. This includes clinical trials for novel therapeutics, natural history studies and the development of improved outcome measures for neurogenetic conditions.
*Please note that RARE-X Exchange Forum sessions require an additional registration fee.
Yssa DeWoody, Ph.D.
Ring14 USA
Yssa DeWoody, Ph.D.
Ring14 USA
Cofounder, Director of Research, and Past President
Ring14 USA
Session:
Integrating Clinical and Patient Reported Data
RARE-X Exchange Forum*
Yssa DeWoody, PhD, is the Cofounder, Director of Research, and past President of Ring14 USA, a non-profit focused on improving the lives for those impacted by neurodevelopmental disorders on the 14th chromosome. This work is a labor of love motivated by her daughter, Marie, who was born with the ultra-rare Ring Chromosome 14 Syndrome. Yssa is a true believer in collaboration as actualized by her commitment to several consortiums including Ring14 International (Cofounder and Past President), the Rare Epilepsy Network (ex officio Chair), Epilepsy Leadership Council, Epilepsy Learning Healthcare Systems, and the Commission for Neurodevelopmental CNVs (founding partner).
*Please note that RARE-X Exchange Forum sessions require an additional registration fee.
Jennifer Farmer
Friedreich’s Ataxia Research Alliance
Jennifer Farmer
Friedreich’s Ataxia Research Alliance
Chief Executive Officer
Rethinking Clinical Trials: What’s Doable? What’s Approvable?
Jennifer Farmer is the CEO of the Friedreich’s Ataxia Research Alliance (FARA). Jennifer has a master’s degree in Genetic Counseling and before joining FARA in 2006, she worked at the University of Pennsylvania and Children’s Hospital of Philadelphia. As the CEO, she helps carry out the strategic mission of the organization through leading FARA’s research and partnership initiatives.
Benjamin Forred, MBA, ACRP-CP
Sanford Research
Benjamin Forred, MBA, ACRP-CP
Sanford Research
Director, Translational Research & The CoRDS Registry
Sanford Research
Session:
The Critical Need for Patient-Led Data Initiatives: Does Size Matter?
Ben has worked in the field of biomedical research since 2009. His experience ranges from nearly a decade working at the bench in basic science, to five years working in the clinical research space. Currently, Ben is responsible for translational research projects at Sanford Research. His team maintains a colony of transgenic mice and a vast number of patient and rodent cell lines that model a number of rare disorders. His group then partners with industry groups to screen promising therapeutics.
Additionally, Ben and his team assist basic scientists with human subject research projects and coordinate clinical sample collection. Ben has also been responsible for the growth of the CoRDS Registry since 2016. CoRDS is an international, disease agnostic, rare disease registry offered at no cost to people living with rare conditions or to the researchers investigating rare disease. Through this endeavor, Ben has built strong relationships with a number of advocacy organizations, and he constantly works to put the wellbeing of rare patients at the forefront of all phases of his career.
Alexandra Gillett, Ph.D.
Wiedemann-Steiner Syndrome Foundation
Alexandra Gillett, Ph.D.
Wiedemann-Steiner Syndrome Foundation
Board Member,
Wiedemann-Steiner Syndrome Foundation
Session:
Partnerships in Action
RARE-X Exchange Forum*
Alexandra Gillett, Ph.D. is a WSSF Board Member since 2024, assisting on the Research and Development Committee. Her son was diagnosed with WSS in September 2021. She has since sought to gather information on WSS and participate in studies that help the community such as participating in and promoting RARE-X.
*Please note that RARE-X Exchange Forum sessions require an additional registration fee.
David Jacoby, MD, PhD
BioMarin
David Jacoby, MD, PhD
BioMarin
BioMarin Fellow in Clinical Science
Vice President, Head of Discovery Medicine
Session — The Critical Need for Patient-Led Data Initiatives: Does Size Matter?
David Jacoby, MD, PhD, joined BioMarin in 2012. Dr. Jacoby leads efforts to identify and evaluate potential clinical indications and technical platforms for early-stage development. Previously at BioMarin, he led development stage programs for hemophilia A, achondroplasia, PKU and CLN2 disease and supported decision-making for non-progressed programs.
Dr. Jacoby trained at Massachusetts General Hospital and Harvard University, with fellowships in movement disorders and molecular neurogenetics. He attended Swarthmore College for his BA and earned an MD and PhD in molecular biology from the University of Pennsylvania. In addition, he is a member of National Institute of Neurological Disorders and Stroke (NINDS) Study Sections on Friedreich’s ataxia and Rare Disease Clinical Trial Preparedness.
Casey McPherson
To Cure A Rose Foundation
Casey McPherson
To Cure A Rose Foundation
CEO
To Cure A Rose Foundation
Session:
What Will $100K Buy You? Emerging Commercial and Non-Profit Financing Models
Casey McPherson is the Founder and President of To Cure a Rose Foundation, and Founder of Everlum Bio. Casey spent his career as a musician; touring the world and releasing two #10 hits in the US. Everything changed when his daughter was diagnosed with a rare genetic disease: HNRNPH2. Casey started To Cure a Rose Foundation to save his daughter Rose, and scale a better model for rare drug development. He then co-founded Everlum Bio, a personalized medicine company and proof of concept drug disco
Manoj Malhotra, M.D.
Ovid Therapeutics
Manoj Malhotra, M.D.
Ovid Therapeutics
Chief Medical Officer
Ovid Therapeutics
Session:
Interactive Groups on Rethinking Clinical Trials
Manoj Malhotra, M.D., Chief Medical Officer at Ovid Therapeutics, leads efforts to develop new medicines for resistant epilepsies. He brings expertise from roles at Eisai, Mallinckrodt and Takeda. A neurologist trained at the Cleveland Clinic Foundation, he also served as Chief of Neurology at Kaiser Permanente. Dr. Malhotra’s dual experience as a treating physician and as a pharmaceutical executive brings deep medical expertise to Ovid and the community we strive to serve.
Katherine Maynard
PWR
Katherine Maynard
PWR
Partner
PWR
Session — Rethinking Clinical Trials: What’s Doable? What’s Approvable?
Katherine Maynard is a healthcare communications strategist with extensive experience in alliance development, media relations, issues management, and brand positioning. For more than two decades, she has built connections with patient advocates, academia, and the healthcare industry to create innovative education initiatives that translate emerging scientific developments and communicate the patient experience. Maynard is currently a partner at PWR.
Dominique Pichard
National Center for Advancing Translational Sciences (NCATS), NIH
Dominique Pichard
National Center for Advancing Translational Sciences (NCATS), NIH
Director, Division of Rare Diseases Research Innovation
Closing Keynote and Remarks
Dominique C. Pichard is the director of NCATS’ Division of Rare Diseases Research Innovation, where she leads rare disease research efforts, collaborating with other divisions and offices across NCATS and other centers regarding rare diseases research. Before joining NCATS, she was the chief science officer at the International Rett Syndrome Foundation. She created several initiatives to improve translational research in Rett syndrome, providing the grounds for successful drug development.
Pichard’s daughter lives with Rett syndrome, so she has a deep understanding of the many challenges associated with having a rare disease, including the diagnostic odyssey and the lack of treatments. Pichard received her M.D. from Georgetown and her M.S. from the University of Maryland School of Medicine.
Charlene Son Rigby
Global Genes
Charlene Son Rigby
Global Genes
Sessions:
* Welcome and Opening Keynote: Shifting the Paradigm to Push Past Limits
* Opening Remarks
* Closing Keynote and Remarks
Charlene Son Rigby has spent her career building organizations at the intersection of data, technology, and life sciences. She currently serves as CEO of Global Genes. She was previously Chief Business Officer at Fabric Genomics and held executive roles at enterprise software and genomics companies, including Oracle and Doubletwist. She started her career in neuroscience research at Roche. When Charlene’s daughter was diagnosed with a rare genetic disease, she co-founded the STXBP1 Foundation. Charlene’s unplanned connection between her personal life and profession has helped push forward the search for a cure for her daughter and kids like her.
Cynthia Rothblum-Oviatt, Ph.D.
FDA Rare Diseases Team
Cynthia Rothblum-Oviatt, Ph.D.
FDA Rare Diseases Team
External Engagement Lead
Session:
Rethinking Clinical Trials: What’s Doable? What’s Approvable?
Interactive Groups on Rethinking Clinical Trials
Dr. Cynthia Rothblum-Oviatt serves as the External Engagement Lead for FDA’s Rare Diseases Team within the Office of New Drugs in the Center for Drug Evaluation and Research (CDER). Prior to joining FDA, Dr. Rothblum-Oviatt spent approximately 20 years in the non-profit rare disease space. For 18 of those years, she was the Science Coordinator for the Ataxia Telangiectasia (A-T) Children’s Project. Dr. Rothblum-Oviatt earned her Ph.D. in biochemistry from the George Washington University.
Tania Simoncelli
Science in Society, Chan Zuckerburg Initiative
Tania Simoncelli
Science in Society, Chan Zuckerburg Initiative
Vice President
Science in Society, Chan Zuckerburg Initiative
Session:
Welcome and Opening Keynote: Shifting the Paradigm to Push Past Limits
Tania Simoncelli joined the Chan Zuckerberg Initiative in 2017 with a vision to build a program committed to centering patients in the biomedical research ecosystem. In 2019, she launched the Rare As One Project – a first-in-kind program that supports patient communities in their quest to accelerate research through grantmaking, capacity building, and the development of tools and partnerships. Her commitment to this work is grounded in twenty years of experience in science policy and advocacy.
Zohreh Talebizadeh, PhD
Global Genes
Zohreh Talebizadeh, PhD
Global Genes
Senior Director, RARE-X Research Program
Global Genes
Session:
The Critical Need for Patient-Led Data Initiatives: Does Size Matter?
Zohreh Talebizadeh leads the RARE-X Research Program as Senior Director and serves as the Principal Investigator for RARE-X. Over the past two decades, her research endeavors have focused on neurodevelopmental disorders, genetics, epigenetics, data science, and patient-centered outcomes research. Her collaborative spirit shines through her strong partnerships with diverse stakeholders, including patient advocates. With over 15 years of experience leading multidisciplinary research projects, Zohreh’s passion for patient-centered research led her to launch a unique initiative promoting the integration of patient perspectives in genetics research.
Zohreh earned her PhD in Genetics from the University of Nebraska Medical Center in Omaha, NE, where she focused her research on the genetics of hearing loss. As a postdoctoral fellow at Children’s Mercy Hospital (CMH) in Kansas City, MO, she deepened her expertise in rare genetic disorders related to neurodevelopmental conditions. Prior to her current role at Global Genes, she served as a Translational Research Manager at the American College of Medical Genetics and Genomics, contributing to the NICHD-funded Newborn Screening Translational Research Network program.
Beyond her professional pursuits, Zohreh has dedicated her personal time and resources to championing human rights causes, amplifying the voices of the marginalized. This has deepened her appreciation for the significance of prioritizing community needs and fostering equity and diversity.
Ramona L. Walls, PhD
Critical Path Institute (C-Path)
Ramona L. Walls, PhD
Critical Path Institute (C-Path)
Executive Director of Data Science
Session — The Critical Need for Patient-Led Data Initiatives: Does Size Matter?
Dr. Walls oversees development of C-Path’s Data and Analytics Platform, expansion and modernization of C-Path’s data integration pipeline to encompass new data types such real world data, and development of a rare disease knowledge graph. Before joining C-Path, she was Assistant Research Professor for Bio5 Institute at the University of Arizona, where she focused on semantic data integration, ontology design, and management of large and dispersed datasets.
Dr. Walls is on the steering committee of the Clinical Research Data Sharing Alliance (CRDSA), is a board member and active technical contributor to the Genomics Standards Consortium (GSC), and is a founding member of the OBO Foundry Operations Committee. She attended Penn State and earned a PhD from Stony Brook University. Dr. Walls is passionate about data science, data integration, data management, and using those skills to make the world a better place.
Ashley Winslow, PhD
Odylia Therapeutics
Ashley Winslow, PhD
Odylia Therapeutics
CEO & CSO
Session: What Will $100K Buy You? Emerging Commercial and Non-Profit Financing Models
Ashley Winslow is CEO and CSO of Odylia Therapeutics, a nonprofit biotech focused on accelerating drug development for rare diseases in partnership with patient groups. Ashley brings to Odylia more than 15 years of drug development experience, with previous positions at the University of Cambridge, Mass General Hospital/ Harvard, Pfizer, and the Orphan Disease Center. She serves as a scientific advisor to the Angelman Syndrome INSYNC-AS council, CHAMP1 Research Foundation, and Global Genes.

Expert Office Hours
Get answers to your questions by scheduling a one-to-one session with one of our experts on topics such as data collection, research strategy, therapy development, and engaging with the FDA. Appointments will open in March for registered attendees.
Tuesday, April 30, 1pm & 4:30pm
*Not all experts are available for all office hours.
Resource Fair
Wednesday, May 1, 2024
1:00 – 2:30 pm
The Resource Fair is designed to help rare disease community leaders explore partnering options for laboratory, gene therapy, cell therapy, biological modeling, data collection and computational services.
Through informal, small-group conversations with clinical and research service providers and peers, rare disease leaders will get answers to their questions and gain knowledge of options available so they can make informed decisions, choose the best partner for their needs, and put their research strategies into action.


Plan Your Visit
Should you need hotel accommodations, please use the information below to book your reservation.
Sheraton Philadelphia Downtown
201 North 17th Street
Philadelphia, Pennsylvania 19103
The conference room block has closed. Please check with the hotel for current rates and availability.
Have questions about this event?
Email [email protected].
Exhibitor Booths
An opportunity to connect and engage with more than 200 advocates, patients, researchers, and partners in the rare disease community.
Learn More Become an Exhibitor
Advocate Support
Applications for stipends are now closed
Support is available to individual patients, close family and friend advocates in the RARE disease community, or staff (paid/volunteer) of RARE disease nonprofit organizations or support groups.
Domestic and international individuals are eligible to receive Advocate Support.
Please note: It is likely that the amount of the Advocate Support will not fully cover all costs associated with the symposium. If you receive support, you will still be responsible for booking your own travel and lodging.
Posters
Posters will be available to view on Monday, April 29th
Sponsors
Interested in supporting the 2024 RARE Drug Development Symposium?
Gold Sponsor

Silver Sponsors




Bronze Sponsors


Partner Sponsors






Looking Back: 2023 RARE Drug Development Symposium
Watch videos from last year’s sessions, or take a peek at the agenda.
RARE Drug Development Symposium Advisory Council
Benjamin Forred, MBA, ACRP-CP
Sanford Research
Benjamin Forred, MBA, ACRP-CP
Sanford Research
Director, Translational Research & The CoRDS Registry Sanford Research Session: The Critical Need for Patient-Led Data Initiatives: Does Size Matter? Ben has worked in the field of biomedical research since 2009. His experience ranges from nearly a decade working at the bench in basic science, to five years working in the clinical research space. Currently, Ben is responsible for translational research projects at Sanford Research. His team maintains a colony of transgenic mice and a vast number of patient and rodent cell lines that model a number of rare disorders. His group then partners with industry groups to screen promising therapeutics. Additionally, Ben and his team assist basic scientists with human subject research projects and coordinate clinical sample collection. Ben has also been responsible for the growth of the CoRDS Registry since 2016. CoRDS is an international, disease agnostic, rare disease registry offered at no cost to people living with rare conditions or to the researchers investigating rare disease. Through this endeavor, Ben has built strong relationships with a number of advocacy organizations, and he constantly works to put the wellbeing of rare patients at the forefront of all phases of his career.Maya Chopra, MBBS, FRACP
Boston Children’s Hospital
Maya Chopra, MBBS, FRACP
Boston Children’s Hospital
Dr. Chopra is a Clinical Geneticist and Pediatrician, having obtained her qualifications in Australia in 2010 through the Royal Australasian College of Physicians and the Human Genetics Society of Australasia. Her expertise is in the assessment and diagnosis of children with rare genetic disorders. In her role as Director of Translational Genomic Medicine at the Rosamund Stone Zander Translational Neuroscience Center (Boston Children’s Hospital, Dr. Chopra is focused on rare neurodevelopmental disorders, particularly in understanding the genotypic and phenotypic spectrum and the underlying mechanisms to inform therapeutic development.
Gabrielle Rushing, PhD
CSNK2A1 Foundation
Gabrielle Rushing, PhD
CSNK2A1 Foundation
Senior Program Director
CSNK2A1 Foundation
Gabrielle Rushing, PhD, is the Science Program Director at the CSNK2A1 Foundation, a non-profit patient advocacy group dedicated to finding a cure for Okur-Chung Neurodevelopmental Syndrome (OCNDS). She completed her PhD at Vanderbilt University, studying how neural stem cells contribute to brain tumor development. Since joining the foundation in 2023, she has developed a research roadmap for OCNDS, established 7 new partnerships, authored a NORD rare disease report, added 2 members to the scientific advisory board, and represented the foundation at 7 events.
Jeanette McCarthy, MPH, PhD
Precision Medicine Advisors
Jeanette McCarthy, MPH, PhD
Precision Medicine Advisors
Jeanette McCarthy, MPH, PhD
Founder
Precision Medicine Advisors
Jeanette McCarthy is a genetic epidemiologist with over 30 years of experience developing products and services that advance the field of genomics and precision medicine. She is an internationally-recognized leading educator, providing technical trainings on clinical genomics to learners around the world and helping numerous organizations educate their workforce and customers to be able to effectively develop and utilize genetic testing in clinical practice.
Amy Raymond, PhD, PMP
Worldwide Clinical Trials
Amy Raymond, PhD, PMP
Worldwide Clinical Trials
Amy Raymond, PhD, PMP
Therapeutic Strategy Lead
Worldwide Clinical Trials
Amy Raymond, PhD, PMP, has been a drug discovery and development professional for 25+ years, including training in molecular biology followed by progressive roles in rare disease clinical operations and clinical strategy for cellular and genetic medicines. Her clinical development experience spans all therapeutic areas and all stages of the clinical development lifecycle. She leverages her combination of training and experience leading the Cell & Gene Therapy Hub at Worldwide Clinical Trials.
Jennifer Tjernagel
SFARI
Jennifer Tjernagel
SFARI
Project Director
Session: What Will $100K Buy You? Emerging Commercial and Non-Profit Financing Models
Jennifer Tjernagel joined the foundation in 2010 to manage the Simons Searchlight program (formerly Simons Variation in Individuals Project) after her previous work as the project manager for the Simons Simplex Collection working through the University of Michigan. Prior to that, she held various roles in the pharmaceutical industry. Tjernagel is a project director at SFARI.
Cara Weismann, Ph.D.
Orphan Disease Center
Cara Weismann, Ph.D.
Orphan Disease Center
Dr. Weismann is the Director for the Program of Excellence (POE) in Mucopolysaccharidosis (MPS) Diseases at the non-profit organization the Orphan Disease Center. Within the POE, she leads a grant program whose goal is to accelerate the development of therapeutics and provide scientific resources to the MPS and lysosomal storage disease communities. She has extensive experience in therapeutic research, drug development, business development, and data sharing policy. She is passionate about using these skills to bring treatments to rare disease patients throughout the world.
