Report Predicts Sharp Jump in Clinical Development Productivity for Rare Disease Therapies
April 23, 2019

A new study from IQVIA Institute for Human Data Science predicts nearly a 30 percent increase in clinical development productivity of rare disease therapies over the next five years, outpaced only by improvements in oncology and neurology.
The report, The Changing Landscape of Research and Development: Innovation, Drivers of Change, and Evolution of Clinical Trial Productivity, examines historical and future clinical trial productivity trends across therapy areas to identify critical productivity changes expected over the next five years.
IQVIA found a record number of new drug approvals in the United States, as 59 novel treatments reached patients. Almost half of these carried an Orphan Drug designation and more than a third were identified as first-in-class with new mechanisms of action.
Although large pharmaceutical companies registered almost half of the new drugs approved in 2018, half of those originated with emerging biopharmaceutical companies, responsible for almost two-thirds of the drug launches. They also account for 72 percent of all late-stage pipeline activity, up from 61 percent a decade ago.
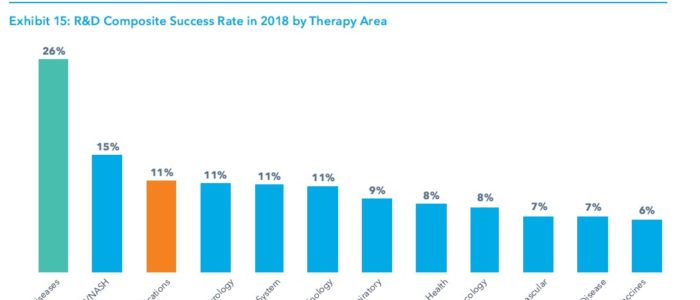
While the overall composite success rate of clinical development stages from phase 1 trials to regulatory submission fell to 11.4 percent in 2018, it remained good for drugs targeting rare diseases, where the rate was 26 percent, more than twice the average.
Successful rare disease drugs had an average 12.2 years of progression time to regulatory approval over the past ten years. This compares to a median 13.6 years for all indications, and is due to a decrease in the regulatory approval timeframe, as rare disease drugs have benefited from a range of novel approval pathways and review mechanisms for orphan indications and breakthrough therapies.
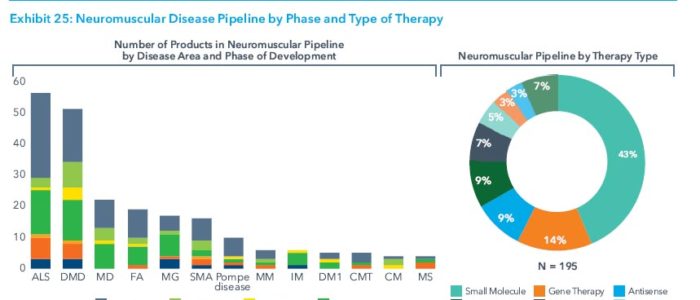
The new drugs also reflect a shift in biopharma R&D from developing therapies that treat symptoms to ones that modify diseases and slow or halt their progression. This is increasingly evident in the neuromuscular pipeline, with its strong focus on experimental rare disease therapies.
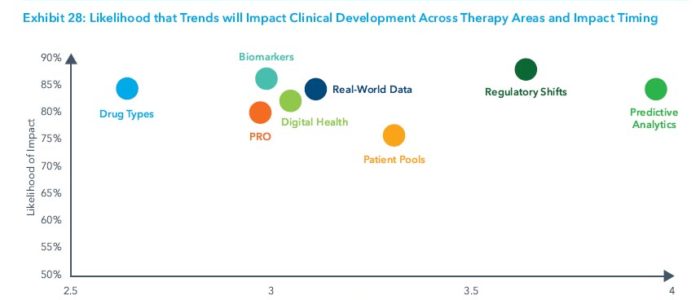
The report identified eight key trends driving changes in clinical trial design, duration, and success:
- Digital health technologies to enable the capture of drug efficacy and safety data remotely, which can improve patient safety, enable virtual trial formats and ease site work burden. They will also make it easier to capture patient-reported outcomes.
- Patient-reported outcomes to shed new light on patient experience, drug efficacy and safety outside the clinical setting and lead to faster trial times as endpoints shift.
- Real-world data to be used to optimize trial design, speed investigator and site selection, and enable new trial designs by acting as virtual control arms and supporting pragmatic, adaptive and real-world evidence registry trials.
- Predictive analytics and artificial intelligence to be used to identify new clinical hypotheses, reduce trial design risks, and speed enrollment by identifying protocol-ready patients.
- Shifts in the types of drugs tested to targeted therapies and disease-modifying treatments to improve efficacy and success rates and accelerate development timelines, but also requiring longer-term patient follow-up.
- Biomarker testing availability to help narrow patient populations to those more likely to see effect, resulting in improvements in efficacy, safety and success.
- Regulatory landscape changes to encourage the adoption of precision medicine approaches, novel trial designs and endpoints while providing means for accelerated drug approvals and regulatory success.
- Pools of pre-screened patients and direct-to-patient recruitment to facilitate enhanced trial enrollment, shortened trial duration, and faster market availability.

Stay Connected
Sign up for updates straight to your inbox.
