Introduction 
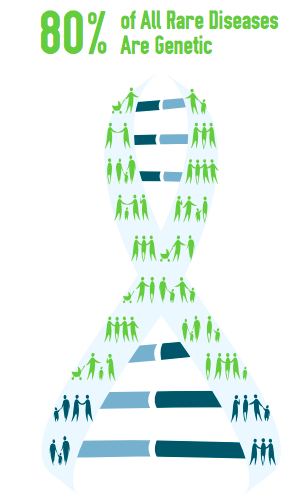
More than 350 million people worldwide suffer from a rare disease. If a disease affects fewer than 200,000 people in the United States, it is considered rare. There are currently about 7,000 rare diseases identified worldwide, and approximately 80 percent are caused by genetic changes. These diseases are often chronic, progressive, complex, life-threatening, and affect the quality of life.
The rapid evolution of technology is accelerating the speed and reducing the cost of genetic testing, making it more accessible to patients. Through genomics, doctors are able to determine the molecular cause of diseases that are oftentimes rare. While it doesn’t always lead to a diagnosis, sometimes doctors are able to identify the root cause of a disease, which can lead to improved treatments. This is still just the beginning of a new age of genomic medicine. As advances occur and a better understanding of the genetic causes of rare diseases are understood, more patients who are undiagnosed today will benefit.
“There’s so much to do on the horizon, to encourage families to persevere and help in terms of supporting their child and determine ways to connect with the scientific community,” says C. Jimmy Lin, President of Rare Genomics Institute. “Now is one of the best times in history to be able to understand these rare diseases and to start to think about developing therapeutics and cures for them.”
This toolkit is designed to provide rare disease patients and their families an introduction to genetics and genetic testing. Advances in genetic testing are rapidly changing the way patients are being diagnosed and treated and providing new hope to patients with rare, genetic diseases.
SECTION 1: Genetics 101 
Each year, many children born in the United States inherit an abnormal gene that will cause them to become ill. In order to find the best diagnostic and treatment options, affected individuals and their families need to understand the basics of the underlying genetics of these diseases.
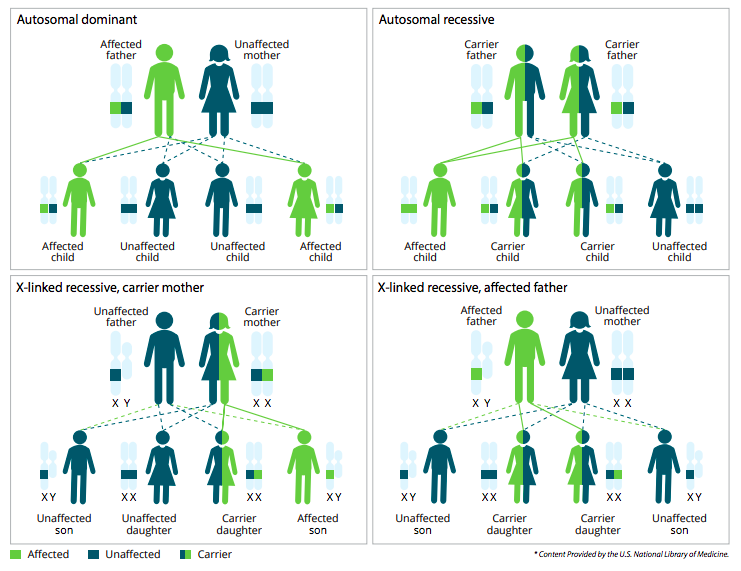
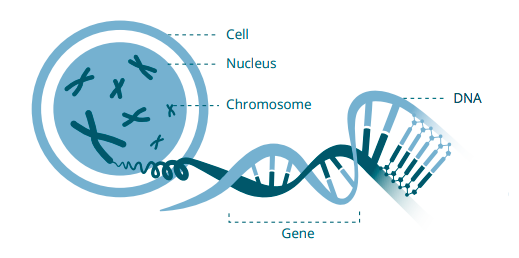
Every person comes with a blueprint, known as the genome, which is contained inside each cell of the body in the form of deoxyribonucleic acid, or DNA. DNA tells each cell what to do and when to play its part. Within cells, DNA is organized into structures called chromosomes. Humans typically have 23 pairs of chromosomes for a total of 46 chromosomes— one set of 23 coming from the father and the other set from the mother. The diagram below shows how the coding for genetic diseases moves from parents to their offspring.
Chromosomes are made up of subunits called genes. A gene is the basic and functional unit of heredity. It provides the necessary instructions for a cell, telling it which specific proteins to produce. These proteins play many roles in the body and can affect physical characteristics, such as hair and eye color, as well as lead to certain genetic diseases.
Genes are made up of introns and exons. Exons are the parts of the gene that are most important for creating proteins. And the human exome is all of the exons in all of the genes, which account for less than 2 percent of an entire genome. Research has often focused on this 2 percent of the genome because it is more feasible to study and easier to compare data against existing research. But there is still a lot we do not understand about the exome.
This genetic information is encoded in genes using a four letter alphabet made up of the letters A, T, C, and G. The letters stand for the molecules adenine, thymine, cytosine, and guanine. These molecules form complimentary base pairs that make-up each of the 3 billion rungs of the twisted ladder that is DNA. When there are mistakes in the sequence of letters, called mutations, this can affect the production of the specific protein that the gene normally encodes. These mutations are either passed down from a parent to a child, or occur randomly in the egg or sperm.
Sometimes, mutations can be beneficial. One example is a mutation in the CCR5 gene. Normally this gene encodes a protein which makes white blood cells susceptible to the HIV viral infection. However, a specific mutation of the CCR5 gene—one that rarely occurs in the population—will give a person partial or complete protection against most forms of HIV. Although mutations can occasionally be beneficial, more often they can cause disease if the error happens in parts of genes that are important for regular body functions.
To learn more about genetics, inheritance, DNA, or genes, the Genetics Home Reference has a handbook dedicated to this subject: http://ghr.nlm.nih.gov/handbookhttp://ghr.nlm.nih.gov/handbook. Additional educational information on rare diseases, genomics, and case studies can be found in the Diagnosing Rare Diseases ebook (www.raregenomics.org/ebooks).
Genetics is complex. It can seem too abstract for some younger children to understand. But parents who want to share this information with their children may consider using GeneEd Web (http://geneed.nlm.nih.gov). GeneEd Web may seem more accessible to younger viewers because it introduces genetic topics, such as cell biology, DNA, genes, and chromosomes, with animation, games, videos, interactive tutorials, and experiment ideas.
SECTION 2: Genetic Testing 
Genetic testing is a type of medical test that identifies changes in chromosomes, genes, or proteins. The results of these tests can confirm or rule out suspected genetic conditions.
If costs were not a limiting factor, every baby’s whole genome could be sequenced (and analyzed) immediately after birth. This would provide all of the baby’s genetic information and make it possible to treat and potentially prevent any diseases that arise. Sequencing every baby’s genome, however, is not currently desirable due to several reasons, including the extremely high cost of testing the entire genome and the fact that most of the genome is still not well understood.
Indications for Genetic Testing
Not every person will need to undergo whole genome sequencing. However, some families may choose to undergo this or other types of genetic testing. Some reasons to consult with a healthcare provider about these types of tests include:
• Personal and/or family history of genetic disease
• Multiple family members with similar symptoms/features without a specific diagnosis
• Symptoms that elude doctors and cannot be diagnosed through continuous testing
• Multiple congenital anomalies/birth defects
• A newborn screening result that indicates a possible genetic disease
• A history of multiple miscarriages and/or stillbirths
• An unexplained infant death
• General developmental delay/intellectual disability
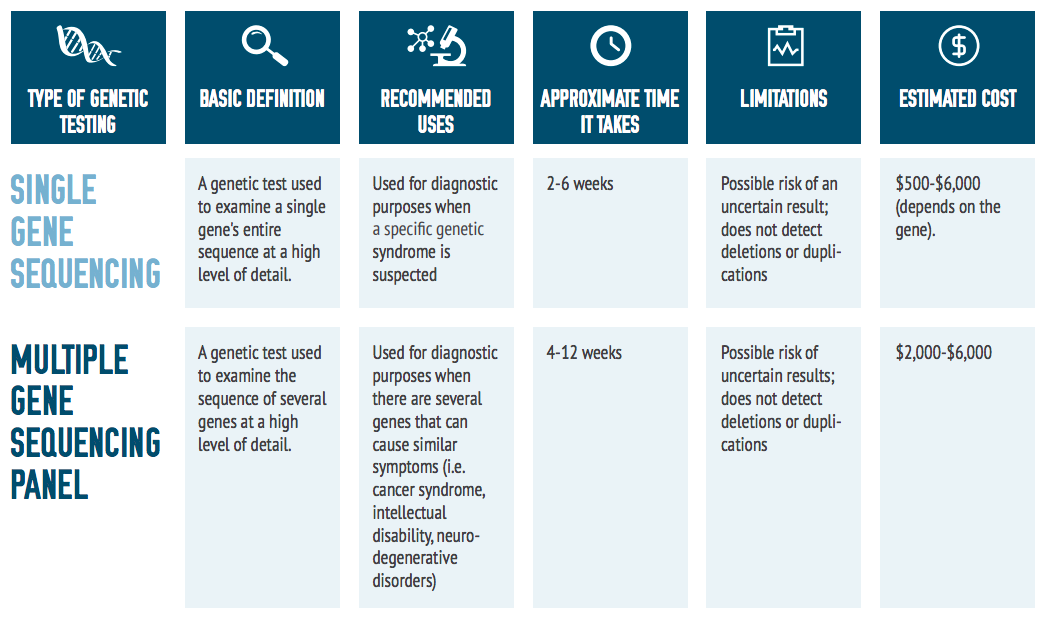
Genetic Testing Options
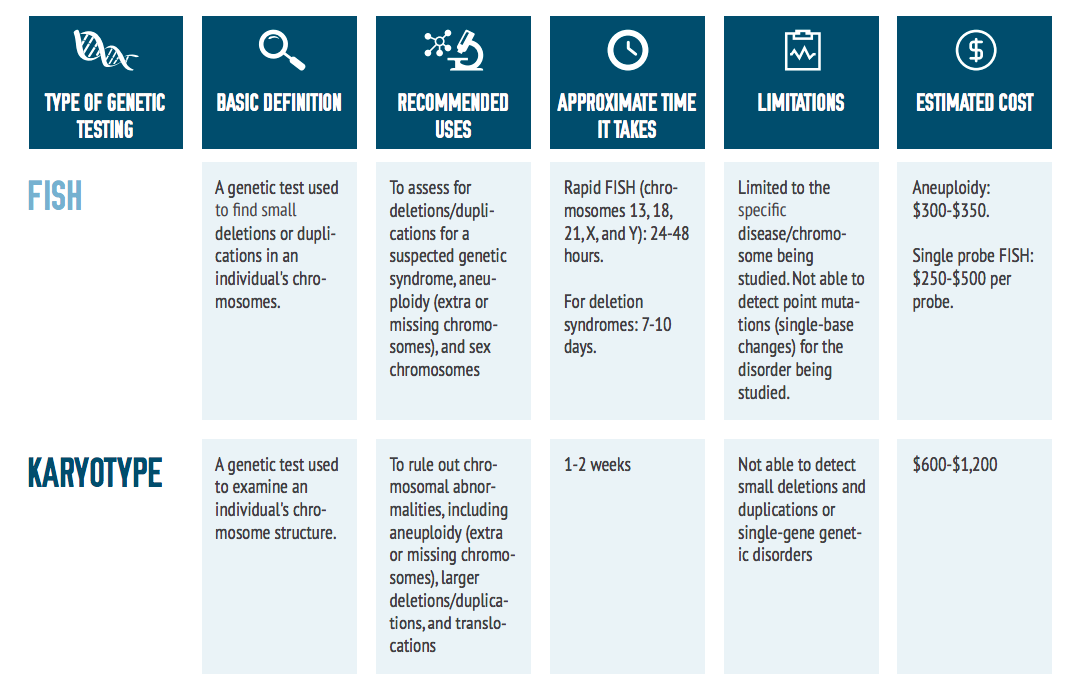
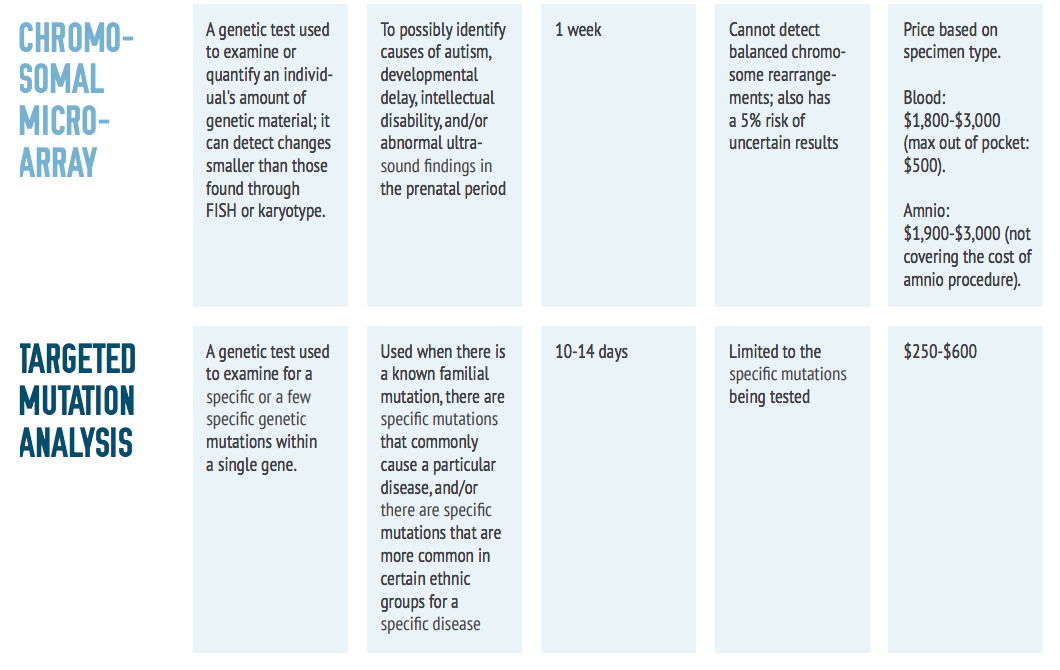
As shown in the comparison chart on the next page, there are many genetic testing options for individuals with rare diseases. Some of the most commonly used tests are described within this toolkit.
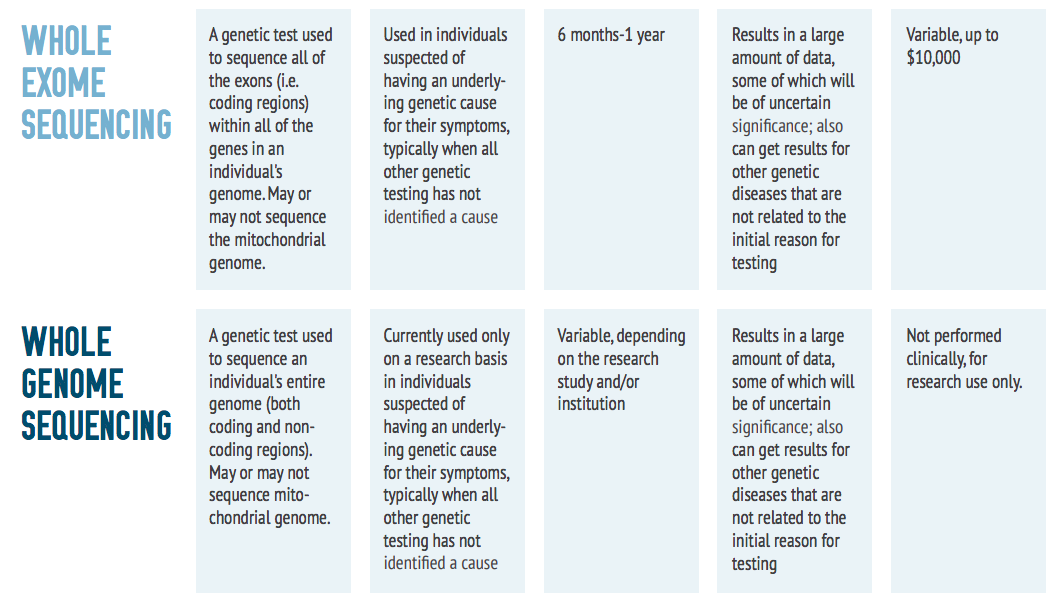
Exome Sequencing
There are several different types of genetic tests available to physicians. A patient’s symptoms and results from prior tests will determine the appropriate one. If prior tests have not revealed conclusive results, a doctor may recommend a whole exome sequencing test.
“Many of our patients have come in with other genetic tests that have been inconclusive or negative. Sometimes families went on a diagnostic odyssey looking for answers for years because ‘there wasn’t a technique to make the diagnosis.’” says Dr. Ada Hamosh, a professor with the Department of Pediatrics and Institute of Genetic Medicine at John Hopkins University School of Medicine. “Now we have that ability with exome sequencing. We needed a technology to say, ‘Aha, this is the diagnosis!’”
This test sequences the exons of about 20,000 genes in the human genome that are responsible for making proteins and contain 85 percent of all known disease-causing mutations. To begin the process, DNA is collected, typically by a blood draw. If a blood draw is difficult, DNA can also be extracted from saliva or a cheek swab. Prior to sample collection, be sure to ask your doctor about preserving the donated sample for future testing. This could help reduce the amount of blood draws, and allows the sample to be used for multiple purposes. Also, inquire as to your rights to a copy of your exome – you should be able to get a copy for your own re-use later.
“People are very generous when they are asked to provide samples for researchers, especially in the rare disease community. However, donating a biological sample such as blood or tissue multiple times can become challenging; it can be a hassle,” says Liz Horn, principal of LHC Biosolutions. “Through Biobanks, biorepositories that store various biological samples and associated information, these samples can be held and used in current and future research studies. It’s a more efficient way to conduct research, and it minimizes the burden on the person donating the sample.”
Results from an exome sequencing test are typically not available for several weeks or months due to the large amount of data the test yields. The results are given to the ordering physician in a report interpreting the sequence information and confirming the presence of any disease causing mutations. This test may or may not be covered by insurance companies.
“About one in three [affected individuals] benefit from exome sequencing, and this test should be used to complement the work performed by the doctor,” says Gary Jackson, a senior clinical account manager at Illumina, a leading developer and manufacturer of genetic sequencing technologies. “Blood work, physical/clinical symptoms, family history, etc. should be analyzed first. Genetics are not the whole picture— just part of the picture.”
Whole Genome Sequencing
If the exome sequencing failed to explain any of the symptoms or features, then whole genome sequencing can be performed. This test will sequence all 3 billion base pairs in the human genome. It also requires DNA to be extracted, preferably through a blood draw. This test is typically only available on a research basis at this time, given the expensive nature of the test and the large amount of data it generates. Results can take several months or even years. Once results are ready, they are returned to the ordering physician in a report containing clinical interpretation of the sequencing data. As with exome sequencing, make sure to ask for a copy of your genome – it’s your data!
Physicians will order this test for patients who have exhausted all other forms of genetic testing. One should note that getting insurance reimbursement for this test is often more difficult than for exome sequencing.
SECTION 3 : MAKING DECISIONS 
Before the genetic test is performed, it is important to understand the testing procedure, the benefits and limitations of the test, and the possible consequences of the test results. The process of educating a person about the test and obtaining permission is called informed consent. This is often conducted by a medical professional (a physician, genetic counselor, genetic nurse, etc.).
The following subsections break down the elements of informed consent, but to learn even more about informed consent, the National Human Genome Research Institute’s “Elements of Informed Consent” (http://www. genome.gov/27526659) further explains the elements of this process.
The Risks and Benefits
There are several benefits and risks associated with genetic testing generally, though each test carries its own specifics. Test results can provide a sense of relief from uncertainty, helping people make informed decisions about managing their healthcare. Genetic testing can reveal:
• A diagnosis, if one has symptoms
• If someone is a carrier of a genetic disease (Carriers have an altered gene, but will not display symptoms of the disease; they may, however, pass this altered gene to their children)
• If a person has an inherited disease before symptoms start
Genetic testing can be helpful for many. Consider patients with CADASIL, a rare, inherited stroke disorder. Patients with CADASIL are not supposed to be given the typical treatments when coming into an emergency room with stroke symptoms.
“Our blood vessels are already compromised with the disease, and the treatments can cause a cerebral hemorrhage,” says Janet Mills, secretary and trustee of CADASIL Association. “I imagine there are other rare genetic diseases that have similar precautions regarding treatments in emergency situations.”
Although genetic testing can have beneficial aspects, there are also potential emotional, social, and financial consequences that come with genetic testing as well. People may feel angry, depressed, anxious, or guilty about their results. Furthermore, the possibility of genetic discrimination is also a concern. The Genetic Information Nondiscrimination Act, or GINA, (http://www.genome.gov/24519851) was passed in 2008 to protect individuals from employment or health insurance discrimination. Unfortunately, GINA does not apply to life insurance, long term care insurance, or military personnel. There can also be unintended consequences of genetic testing, such as learning about potential risks for other diseases or information about ancestry that people may not want to know.
“The key in any genetic testing is to understand the choice before deciding whether or not to get the test. Informed consent is not about the absence of risk, but the conscious decision that the benefits are worth accepting them,” says John Wilbanks, the Chief Commons Officer of Sage Bionetworks. “Most genetic testing today is very low risk to most people, and there is a robust genetic counseling community to help you navigate a specific test’s particular benefits and outcomes. Informed consent processes should always help you understand the test at hand and connect to experts who can give even more detail.”
Limitations
Genetic testing can provide only specific information about an inherited condition: the existence of a mutation, or the number of repeats of a mutation, and so forth. But the genotype is not usually a perfect forecast of the body: the test results may not be able to determine if and when a person will show symptoms of a disorder and how severe the symptoms will be.
Another major limitation is the lack of treatment strategies for many genetic disorders once they are diagnosed. In many cases, there are no treatment options for these conditions. However, research is always being done to create or redesign treatments and therapies. A truly informed consent should emphasize understanding this profound limitation.
Opportunities
In very rare cases, treatments already on the market can be rapidly targeted at newly found genetic illnesses. But in most cases, the benefit will be the creation of a knowledge base for the condition under study. This is why it is so important to ask for a copy of your data at all times– it allows you, later, to connect with others to create pooled data for “data scientists” to analyze.
With increasing computational resources, along with new algorithms for querying growing amounts of data, Dr. Barry Bunin, CEO of Collaborative Drug Discovery, says we’re able to find new uses for drugs which are already on the market. This is called drug repurposing, and he says it provides great promise for those with rare disease.
Consulting with Genetic Counselors
A genetic counselor can help you understand the science behind your disease’s inheritance pattern. When the specific chromosome where the mutation is found is known, a counselor can help you understand what has happened in your genetics. “This can be scary and fascinating at the same time, says CADASIL Association’s Mills. “The vagueness of ‘possibly’ inheriting is gone when you are given the odds and realize the chances of having at least one of your offspring diagnosed is fairly likely.”
The National Society of Genetic Counselors defines genetic counseling as “the process of helping people understand and adapt to the medical, psychological, and familial implications of genetic contributions to disease.” People can consult with genetic counselors to understand the scientific, emotional, and ethical factors surrounding the decision to have genetic testing and how to deal with the results of those tests.
“Genetic counselors provide non-directive counseling,” says Andrea Knob, genetic research counselor and coordinator for Beth Israel Deaconess Medical Center. “This means that they help patients understand the information and options available, but they do not advise patients on their decisions. Ultimately, the patient makes their own decisions regarding testing. The genetic counselor is there to provide information, options, and support.”
To locate a genetic counselor, use the search engine on the American Board of Genetic Counseling website (https://abgcmember. goamp.com/Net/ABGCWcm/Find_Counselor/ ABGCWcm/PublicDir.aspx?hkey=0ad511c0- d9e9-4714-bd4b-0d73a59ee175) or the “Find a Genetic Counselor” tool on the National Society of Genetic Counselors website (http:// nsgc.org/p/cm/ld/fid=164).
Getting Access to Testing In many cases, health insurance plans will cover the costs of genetic testing when it is recommended by a person’s doctor. The decision is dependent on the specific test, the reason for the test, and the insurance provider. Note: Not all clinical tests are covered by insurance.
Health insurance providers have different policies about what tests are covered. Contact your insurance company before pursuing genetic testing to ask about coverage.
Before contacting the health insurance provider, be prepared to have:
• The name of the test
• The name of the laboratory the test will be performed at
• The CPT (current procedural terminology) codes
In addition to asking about the insurance coverage of the test, the patient should also consider asking about out of pocket expenses. Some policies might only cover a portion of the cost, leaving the remainder for the patient’s family to take care of.
One way to offset the out of pocket expenses of genetic testing is crowdfunding, a method of raising awareness and capital through the internet and social media. Crowdfunding gives people the opportunity to read your story, spread awareness, and support your cause.
A few crowdfunding options to explore include:
• Consano (https://www.consano.org/): A nonprofit that focuses specifically on medical research, Consano is a platform that enables users to donate directly to medical research projects. Consano funds its overhead from corporate partnerships, foundation grants, and private donations.
• FundRazr (https://fundrazr.com/): This platform helps users raise money for matters important to them. Setting up a campaign is easy and free.
• GiveForward (http://www.giveforward. com/): This site allows users to create and share fundraising campaigns easily.
• GoFundMe (http://www.gofundme.com/): GoFundMe lets users set up a free, customizable fundraising site in a few minutes.
• IndieGoGo (http://www.indiegogo.com/): This platform helps users raise money for all types of campaigns.
• Rare Genomics Institute (http://raregenomics.org/): A nonprofit that provides an expert network and an online crowdfunding mechanism, Rare Genomics Institute brings together scientists and leverages the crowdfunding capabilities of the internet to bring hope to patients.
• YouCaring (http://www.youcaring.com/): Setting up a profile page and raising funds through YouCaring is simple. It provides easy-to-follow steps on how to get everyone started.
There are a few topics you may want to consider before you set up your own customized microsite. Romina Ortiz, the Vice President of Policy and Patient Advocacy at Rare Genomics Institute, says in order to be effective at raising funds, it is important to tell your story as a family member, explaining how the funds are needed to cover genetic testing for a loved one with an undiagnosed rare disease. Make sure to express how you care about your loved one’s health and future.
“Being comfortable with explaining this,” says Ortiz, “will go a long way toward communicating the value and change that your donors and supporters will be giving.”
Additional tips on raising money can be found within the 5 Essential Tips & Tools for Effective Fundraising Toolkit. (http://globalgenes.org/toolkits/5-essential-tipstools-for-effective-fundraising/introduction/).
Appendix: Finding Answers through Genetic Testing 
Kim Miller, Mother of Jasey
When our daughter Jasey was born, like most parents, it was a time of joy and excitement. However, happiness was quickly overshadowed by tears, questions, and prayer early in her life. When she was just a week old, Jasey stopped breathing and had to be hospitalized.
We spent a few weeks in the hospital as the doctors worked to determine why she continued to stop breathing. She would be released from the hospital—only to return a few days later and stay for a month. Jasey stopped breathing nine times within the first two weeks of her life. She was diagnosed with seizures and was placed on a seizure medication and a breathing monitor.
As Jasey continued to get older, we noticed that she was not hitting milestones that other children her age were reaching. She began physical therapy and then added speech and occupational therapies to help with her developmental delays. In addition to developmental delays, she was diagnosed with failure to thrive, meaning her growth and weight gain were not what they should be.
Her pediatrician referred us to a local hospital for genetic testing. Jasey went through numerous genetic tests over the next few years of her life. Nevertheless, we were still without an answer. I started researching children’s hospitals that specialized in genetic research.
I contacted Cincinnati Children’s Hospital, where they performed an MRI on Jasey’s brain and whole exome sequencing. Exome sequencing identified a mutation in the gene known as PACS1, a rare genetic mutation—so rare, in fact, that Jasey was only the third person in the world to be diagnosed with this mutation at the time.
Jasey continues to receive physical therapy, speech therapy, and occupational therapy. She is making great progress. She is a walking miracle. We are continuing to work with Cincinnati’s Genetics Department in our quest to learn more about this mutation.
Takeaways from the Author:
1. Exome sequencing made diagnosis possible. Without this test, we would still be searching for answers and constantly justifying why Jasey needs various therapies.
2. Exome sequencing can be very time consuming, but it is well worth the wait. Since receiving Jasey’s diagnosis, we have been able to communicate with other parents whose children have recently been diagnosed with the same mutation. Communicating with others in similar situations has been very helpful and invaluable.
3. Prior to receiving a diagnosis, most of Jasey’s doctor appointments required blood draws and tests. Now we can focus on learning more about the genetic mutation and how to best help her.
Resource guide 
Crowdfunding for Medical Support
Consano (https://www.consano.org/): A nonprofit that focuses specifically on medical research, Consano is a platform that enables users to donate directly to medical research projects. Consano funds its overhead from corporate partnerships, foundation grants, and private donations.
FundRazr (https://fundrazr.com/): This platform helps users raise money for matters important to them. Setting up a campaign is easy and free. GiveForward (http://www.giveforward.com/): This site allows users to create and share fundraising campaigns easily.
GoFundMe (http://www.gofundme.com/): GoFundMe lets users set up a free, customizable fundraising site in a few minutes. IndieGoGo (http://www.indiegogo.com/): This platform helps users raise money for all types of campaigns.
Rare Genomics Institute (http://raregenomics.org/): A nonprofit that provides an expert network and a

Stay Connected
Sign up for updates straight to your inbox.