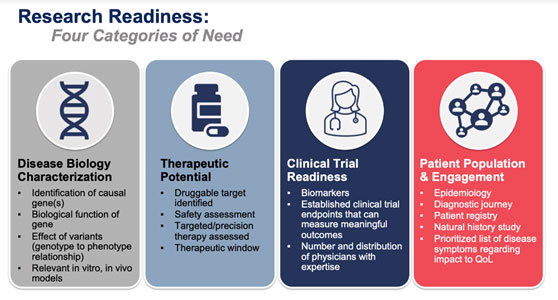
In May 2023, Global Genes and the Orphan Disease Center of the University of Pennsylvania hosted the RARE Drug Development Symposium and discussed using new technologies in early stage research, including how to know if your organization is “research ready?”
This session closed the conference by bringing together the themes from two-days of conversations around patient-driven drug development, applying lessons learned to the collection and management of patient-collected data.
Notable quotes from session:
Don’t stress about which step to take first – just take one. There is no perfect path. – Sunitha Malepati
There are a number of ways that patient registries can be used beyond just research:
– Share clinical trials & research opportunities
– Raise awareness of the disease and burdens faced
– collect data that is accessible for research
– Understand patient and caregiver perspectives
A global registry is best – In rare diseases, it’s critical to include as many patients as possible, and it’s important to have a more diverse patient population . Only one-third are from the U.S. Keep in mind cultural boundaries when you have a global registry. – Sophia Zilber
Key Takeaways:
- Partner with organizations like ODC’s JumpStart and Global Genes to identify gaps in your research program
- Create a decision tree. What are your goals? Where do you want to be and how fast do you want to get there?
- Remember that no matter which treatment modality your group or a research partner is pursuing, patient data and community engagement are key.
Moderator:
Karmen Trzupek
Sr. Director, Scientific Programs, RARE-X, Global Genes
Panelists:
Sunitha Malepati
VP, CACNA1A Foundation
Ashley Winslow, Ph.D.
President and CSO, Odylia Therapeutics
Sophia Zilber
Patient Registry Director, Cure Mito Foundation

Stay Connected
Sign up for updates straight to your inbox.
