Archived Events
2023 RARE Drug Development Symposium
Global Genes and the Orphan Disease Center of the University of Pennsylvania hosted the RARE Drug Development Symposium in May 2023.
Collaborate — Go Farther, Together
The RARE Drug Development Symposium, hosted by Global Genes and the Orphan Disease Center of the University of Pennsylvania, equips advocates with the knowledge, skills and connections they need to advance therapy development for their communities. The 2023 event focused on collaboration – the foundation of success in rare disease research.
Keep scrolling to see more content from the 2023 event, including videos of the sessions, key takeaways from each session, posters, the RARE Research roadmap, and webinars hosted before and after the event.

Sessions & Key Takeaways from the 2023 RARE Drug Development Symposium
Charting the Path to Treatments
Each path toward development of rare disease therapeutics is different. In this plenary, you’ll hear from advocates who took different paths. Use their insights to get closer to approved treatments and therapeutics for your community.
View SessionRare Research Roadmap
An overview of the end-to-end drug development process highlighting interconnection points where patient advocacy groups can contribute. This video helps you understand the flow of basic research, early-stage research, clinical trials, regulatory, commercialization, and post-market follow up.
View SessionUnderstanding Data for Basic Research
Building a firm foundation for your research program helps avoid wasted time, money, and effort. Why is the data collected and produced by basic research important? What role does it play in driving research strategies?
View SessionNew Technologies in Early Stage Research
Find out how emerging technologies may be used for drug discovery, design and repurposing, transforming an often complex, decades-long mission into a more efficient process, reducing the timeline and cost to bring therapies to patients.
View SessionIntellectual Property: Balancing Stakeholder Rights
The founder of the University of Pennsylvania’s Orphan Disease Center outlines key concepts that advocates should be familiar with when negotiating contracts with industry and academic partners.
View SessionThe Regulatory Landscape: Pathways, End Points, and Clinical Trials
Learn about the different pathways open for rare disease therapeutics, why patients are essential to establishing meaningful outcome measures in these pathways, and what patient advocates can do to influence clinical trial design.
View SessionResearch Readiness
This session brings together the themes from two-days of conversations around patient-driven drug development, applying lessons learned to the collection and management of patient-collected data.
View SessionHow Can the FDA Improve Processes?
CBER Director Peter Marks discusses strategies that regulatory agencies are applying to accelerate treatment development for rare diseases.
View SessionPre-RDDS Webinar
How to Support Your Community Before, During, and After Advanced Therapy Trials
During this session, you will hear from advocacy leaders and a physician on the role advocacy groups can play in anticipation of an advanced therapy clinical trial and how that role changes once the trial is in progress and then completed. You will also learn what makes advanced therapy trials unique and what the experience is like to help make informed decisions.
2023 RARE Drug Development Symposium Posters






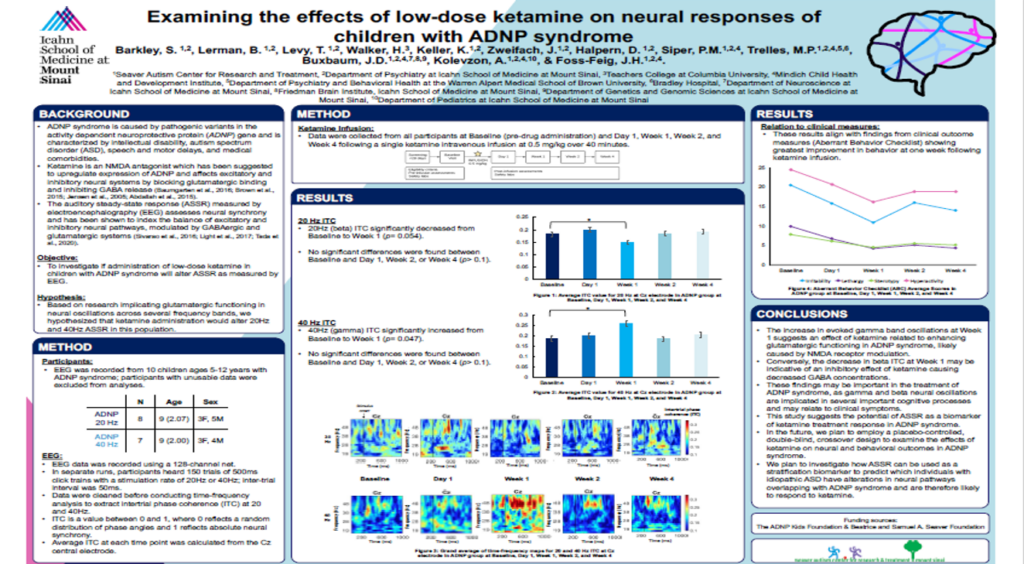
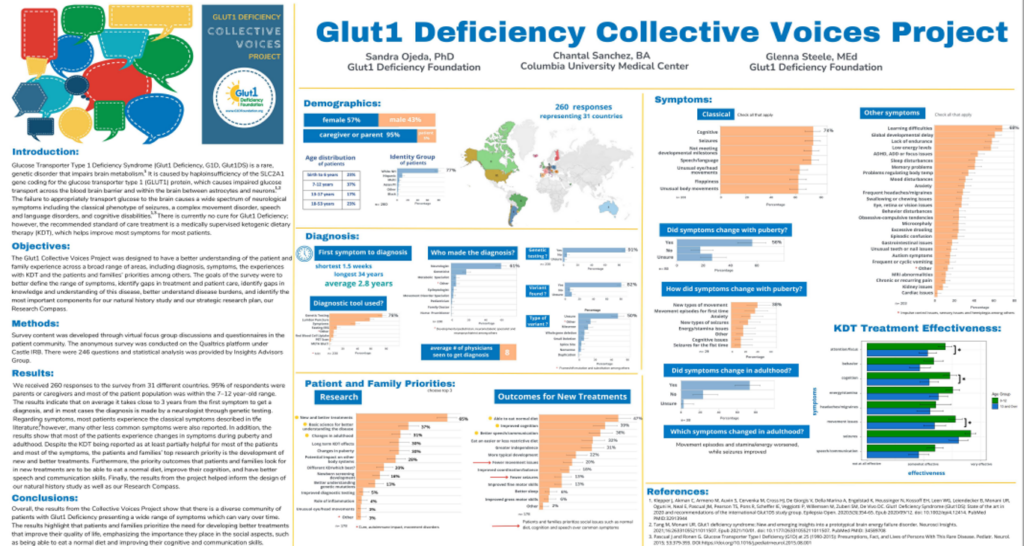
What we learned from the 2023 RARE Drug Development Symposium
2023 Recap
Check out some stats from our event this year.
2023 RARE Drug Development Symposium Stats